

Figure Set 3: Nitrogen Fertilizers Increase Nitrous Oxide Emissions
Cognitive Skills: (see Bloom's Taxonomy) - knowledge, interpretation, synthesis
FACULTY NOTES
In this guided class discussion, the suggested strategy is to show Table 3a to your students, have them interpret the table on their own for a moment, and then discuss the Table as a class using the questions listed in the student instructions as prompts. Instead of asking for a show of hands to answer questions, call on randomly selected students to ensure participation by the entire class. Repeat the same strategy for Figure 3.
Part 1
Students are likely to struggle initially with the term "relative radiative effectiveness." This is a unit-less measure that compares the heat trapping potential of a molecule of different greenhouse gases to a molecule of carbon dioxide, where a molecule of carbon dioxide is set as the baseline. Greenhouse gases are also compared on a volume basis. There are several natural and anthropogenic sources of N2O, which are listed in Table 3b, which is included for faculty reference. Management practices on agricultural soils are the single largest category in terms of N2O emissions, contributing 3.3 Tg N2O-N per year.
Nitrous Oxide Sources |
Tg N2O-N per Year |
Ocean |
3.0 |
Tropical |
|
Wet Forests |
3.0 |
Dry Savannas |
1.0 |
Temperate |
|
Forests |
1.0 |
Grasslands |
1.0 |
Agricultural Soils |
3.3 |
Biomass Burning |
0.5 |
Industrial |
1.3 |
Feedlots |
2.1 |
Total |
16.2 |
| Tg = teragrams (1012 grams) | |
Table 3b. Global nitrous oxide budget based on calculations in 1997.Data are from the Intergovernmental Panel on Climate Change (IPCC) 1997 report.
Part 2
Students may have trouble answering the last question because it is designed to make them think through the problem. Guide them through the question to help them realize that nitrous oxide emissions may be very high during corn crop production, thus off-setting the climate benefits of growing corn as a biofuel. As stated in the background material, a good general source about human alteration of the global nitrogen cycle can be found on the Ecological Society of America website: www.esa.org/science_resources/issues.php.
Nitrous oxide is a kind of by-product of both nitrification and denitrification. Sometimes denitrification does not go all the way to nitrate, with nitrous oxide as the end product instead. The equations for nitrification and denitrification are listed below. The terminology here is very confusing, largely because the terms (e.g. nitrification) do not have an obvious meaning; therefore you need to decide what information you want students to remember, because the details can be overwhelming. It is not necessary that students learn the equations for nitrification and denitrification, but we've supplied the equations for your reference. During nitrification, ammonia (NH3) is converted to nitrate (NO3-). However, nitrous oxide (N2O) is also a minor byproduct of this reaction.
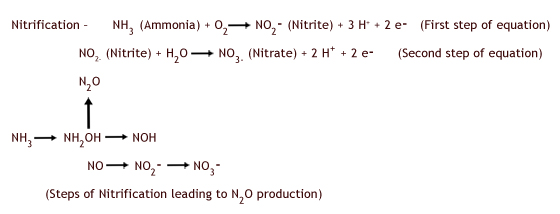
Denitrification, the conversion of nitrate (NO3-) to nitrogen gas (N2), occurs in low oxygen soil conditions. Nitrous oxide is an intermediate in the denitrification process and can be an end product for some bacterial taxa (Robertson and Grace 2004).
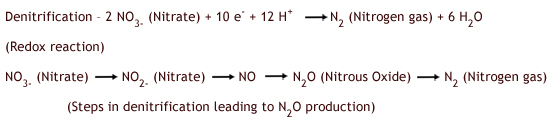
In the nitrogen cycle, bacteria are engaged in very different processes. Nitrification is actually a type of chemoautotrophy in which bacteria use the energy released from oxidation of ammonium to nitrate to reduce carbon dioxide to carbohydrates. In contrast, denitrification is a type of respiration in which carbohydrates are oxidized for energy; in this case it is an anaerobic respiration and nitrate is used instead of oxygen. It may be wise not to tell students this level of detail, but it is good for you to recognize these differences.
McSwiney and Robertson (2005) found that N2O emissions did not increase linearly with nitrogen fertilizer rates, but that "excess" nitrogen fertilizer resulted in substantially larger N2O emissions. Nitrogen is a limiting nutrient for corn crop growth, but the application of nitrogen fertilizer can saturate the supply of nitrogen. Above 101 kg N per hectare, corn crops no longer responded with larger yields, and the supply of nitrogen in the soil was larger than the demand by the corn crops. Excess nitrogen in the soil resulted in much higher rates of N2O production via the nitrification and denitrification processes. Previous studies (Bouwman 1996) had estimated a linear relationship between N2O emissions and nitrogen fertilizer rates (see student assessment).
There appears to be a threshold where crop yields level off above 101 kg N per hectare while N2O emissions increase dramatically. Nitrous oxide emissions were particularly high at 134 kg N per hectare, which cannot be directly explained. It could be due to experimental error, which would be unlikely due to randomization in the experimental design and multiple years of data collection. The authors of Figure 3b suggest a potential change in the microbial process by which N2O is produced, or that another N sink (luxury plant uptake, microbial immobilization) could be competing for nitrogen at the higher N rates. This may be a good discussion point for the class - asking them why N2O emissions were so much higher at 134 kg N than 168 and 202 kg N per hectare.
The assessment below is a short essay that requires students to describe the relationship between nitrogen fertilizer rates and nitrous oxide emissions. A linear equation does not appear to fit the data well, as nitrous oxide emissions are variable and quite high above fertilizer rates of 101 kg ha-1. Students need to understand the equation for a linear model in order to answer the question completely. Introductory students may need some guidance to answer the question, but should be able to provide an answer using basic algebra and geometry.
Post Lesson Assessment - Short Essay (100 - 200 words):
An article published in 1996 provides an equation for estimating nitrous oxide emissions from agricultural fields based on the amount of fertilizer applied (Bouwman 1996). The equation that they use to calculate nitrous oxide emissions is: E = 1 + 1.25F (E = kg nitrous oxide per hectare, F = kg nitrogen fertilizer per hectare).
Would this equation (E = 1 + 1.25F) accurately estimate the amount of nitrous oxide emitted from the fields that were studied to generate the data in Figure 3? Why or why not?