

Figure Set 2: Methane Emissions from Agriculture
Cognitive Skills: (see Bloom's Taxonomy) - Knowledge, Interpretation
FACULTY NOTES
The suggested student active approach suggested for Figure Set 2 is "Think-Pair-Share." This approach is similar to "Turn to your Neighbor" and requires students to think about the question, turn to their neighbor to discuss the question, and then share their answer with the class.
Students may need help understanding some of the terms in Table 1. For instance, relative radiative effectiveness may need to be described as the potential for each of the gases to trap heat in the atmosphere, and thus contribute to global warming. Therefore, one kilogram of methane is 25 times more effective at trapping heat compared to carbon dioxide. Two factors influence relative radiative effectiveness, which are physical chemistry (including radiation absorption properties) and lifetime of a molecule in the atmosphere. Physical chemistry of a molecule determines the infrared (IR) wavelength absorbed. Gases with absorption bands in the non-visible portion of the IR spectrum, particularly between 1,000-1,200 wavenumbers, have the highest radiative forcing effect. Carbon dioxide absorption peaks occur at 2350 and 650 wavenumbers while methane absorption peaks occur at 3,000 and 1,300 wavenumbers.
In order to calculate the answer for part 1, question 3, students must consider not only the ppm increase of CO2 and CH4 in the atmosphere, but also the molecular mass of these gases. This is necessary since relative radiative effectiveness is calculated per unit mass (e.g. per kilogram), and not per molecule.
As stated before, methane absorbs frequencies of IR radiation emitted from the Earth's surface that would otherwise continue out to space. Even though methane is more effective per molecule than carbon dioxide at radiating heat, carbon dioxide is still the most important greenhouse gas because the quantity of carbon dioxide created by human activities is much greater than the quantity of methane. The students are assigned to discuss this with a partner. Continue this discussion with the entire class, to make sure they know that methane is a substantial contributor to climate change, but is still not as important as carbon dioxide.
We've included Figure 2b here for faculty reference. Use Figure 2b in a more advanced class if you wish. Figure 2b describes methane sources and sinks. Methane source and sink values in Figure 2b were calculated in individual studies, and were then compiled and scaled to a global level by Moss et al. (2000). There are slight disagreements between the values in the figure, reflecting the error involved when scaling up from multiple scientific studies. Students may be confused regarding why there is methane left over and it does not all get consumed by the reaction with hydroxyl molecules in the atmosphere. They may also notice that there should be much more methane in excess after considering how much methane comes from human activities. However, the methane sinks (oxidation in the atmosphere by hydroxyl radicals or oxidation in soil by methanotrophic bacteria to CO2 and H2) have the capacity to oxidize some of this excess methane produced by human activities, but these sinks cannot oxidize all of the excess methane. Methane is gradually broken down to CO2 and H2 through a series of chemical reactions, which explains its relatively short life in the atmosphere.
Methanogens are the only living organisms that produce methane as a way of life. The biochemistry of their metabolism is unique and definitively delineates the group. The terminal electron acceptor in methanogenesis is not oxygen, but carbon. The two best described pathways involve the use of carbon dioxide and acetic acid as terminal electron acceptors.
Methanogenesis - CO2 + 4 H2 ? CH4 + 2 H2 or CH3COOH ? CH4 + CO2
Methanotrophs are bacteria that can use methane as their only source of carbon and energy. They are responsible for methane oxidation in soil.
Methane oxidation in soil - CH4 + 2 O2 ? CO2 + 2 H2
Students are asked to consider what individuals might do to cut down on methane emissions. Some examples may include eat less meat or drink less milk. They may also say eat less rice or use less fuel. Answers may vary and, hopefully, will be creative. Bringing in the social dimension will make it more interesting and relevant for students.
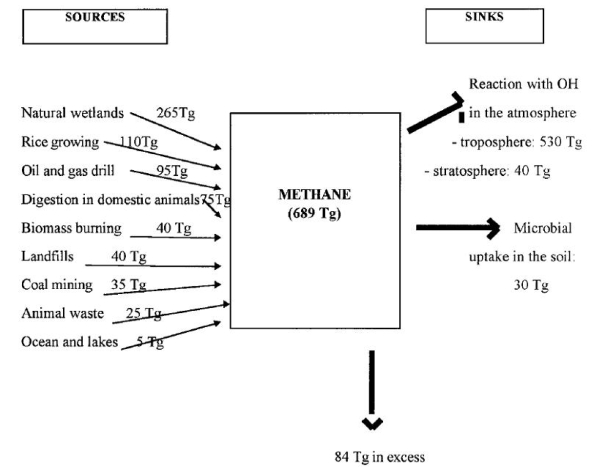
Figure 2b. Methane sources consist of natural and anthropogenic locations. Two major methane sinks exist; oxidation by hydroxyl (OH-) in the atmosphere and oxidation by methanotrophic bacteria in soil. This figure is taken directly from Figure 1 in Moss et al. (2000), which is published in the journal 'Annales de Zootechnie.'
Students should complete the "Minute Paper" below for an assessment of the material presented. Be sure to discuss a couple of the main ideas found in the Minute Paper's during the next class period to show to the students that the Minute Paper is valuable.
Post Lesson Assessment - Minute Paper:
Students take two minutes at the end of the class period to write an answer to the following questions on a piece of paper - to be turned in.
- How do agricultural practices produce methane?
- Why is it important to consider methane as a greenhouse gas when there is 350 times more carbon dioxide in the atmosphere than methane?