Detailed Description of the Experiment (written for students)
Introduction
If individuals of a species are adapted to a particular environment, any change in the environment may lead to reduced fitness. As a result, a rapid evolutionary response to environmental changes can be advantageous. Environmental changes that might lead to an evolutionary response include changes in the local environment, changes in the global environment (e.g., global climate change), or changes in the natural range of environments that a species inhabits due to range expansion.
In phytophagous (phyto=plant, phagous=eating) insects, different species or different populations of the same species are often specific to a particular host plant species (i.e. are specialists). Therefore, a change in the availability of a particular host plant or the introduction of a new host plant may lead to a shift in the host plant used, which in turn could lead to strong natural selection for adaptation to the new host plant. Adaptation after host shift in herbivorous insects has been documented in a wide range of species (Via 1990).In some species, the evolutionary response of insects to a new host can be very rapid. For example, soapberry bugs (Jadera haematoloma) historically used balloon vine (Cardiospermum corindurn) and the soapberry tree (Sapindus saponaria) as their hosts (Carroll et al. 1997). However, in the 1950s, the goldenrain tree (Koelreuteria elegans) was introduced into Florida. By 1990, soapberry bugs that had switched to using goldenrain trees as a host had evolved shorter beaks. In addition, when soapberry bugs from both balloon vine and goldenrain tree were reared on goldenrain tree, those that had switched to using goldenrain trees were larger and developed more rapidly on goldenrain (Carroll et al. 1997). Similarly, in the checkerspot butterfly Euphydryas editha, females evolved a preference for a novel host and rejected their native host in just seven years (Singer et al. 1993).
Bean beetles (cowpea seed beetles), Callosobruchus maculatus, are agricultural pest insects of Africa and Asia. Females lay their eggs on the surface of beans of several species in the family Fabaceae. Although bean beetles are generalists, females prefer to lay eggs on their natal host (Messina 2004a).Eggs are deposited (oviposition) singly. Several days after oviposition, a beetle larva (maggot) burrows into the bean and cannot move from the bean on which an egg was deposited. As a result, the quality of the food resources available in a bean will influence the developing individual’s growth, survival, and future reproduction (Mitchell 1975, Wasserman and Futuyma 1981). At 30°C, pupation and emergence of an adult beetle occurs 25-30 days after an egg is deposited, completing one generation of the life cycle. Adults are mature 24 - 36 hours after emergence, and they do not need to feed. Adults may live for 1-2 weeks during which time mating and oviposition occurs. Because the ability to use the resources of the host bean efficiently is important in determining larval growth, survival, and future reproduction, we would expect populations to adapt rapidly to the host plant species that are available.
Materials and Methods
Overview of Data Collection and Analysis Methods:
Week 1
In class, you will be provided with live cultures of bean beetles containing adults that have been raised on mung beans (Phaseolus aureus) for a large number of generations and other bean beetle cultures that were originally from mung beans but were switched to adzuki beans (Phaseolus angularis) only 2-3 generations ago. Supplies of organic mung and adzuki beans also will be available.(Note to instructors: The preceding sentences should be changed depending on the alternative host species and how long ago beetles were switched to that host.) Female beetles are easily identified in the live cultures because they have two dark stripes on the posterior of the abdomen, whereas the posterior abdomen of males is uniformly light in color (Figure 1).
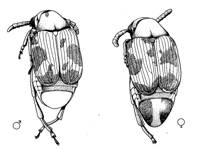
Figure 1. Dorsal view of male and female bean beetles (Callosobruchus maculatus).The sex specific coloration of the posterior abdominal plate (pygidium) is shown (Figure from Brown and Downhower, 1988).
Prior to the laboratory class, each student should design an experiment or set of experiments to address whether rapid local adaptation has occurred in the bean beetle cultures that were recently switched to adzuki beans. Each individual will discuss his or her experimental design with others in a small group, and each group will present a consensus design to the class. Based on the experimental designs presented by the groups, we will discuss common experimental approaches for the entire class.
After you have read the background information and before the laboratory class meeting:
- Describe at least one experimental design for evaluating whether local adaptation to host species has occurred.
- Predict the outcomes for the experiment.
- List the dependent variables you would measure to determine if your predictions were true.
- Identify and list the variables you would manipulate in each experiment.
- Identify and list the variables you would keep constant in each experiment.
- Describe the statistical analyses that you would carry out to test your predictions.
Come to class prepared to present your experimental designs. Each individual will share his or her experimental design with their group, and then the group will present their consensus experimental design to rest of the class. Together, we will develop a class-consensus experimental design. Based on this experimental design, each person should set up one replicate for each treatment. We will pool the data from the entire class for analysis.
Week 2
(Note: this information wouldn’t be given to the students because it would provide too much information about experimental design)
Each student should check their replicates for beans with single eggs. Each bean with a single egg should be placed in its own Petri dish or well of a tissue culture plate. At a minimum, the host of the female that laid each egg should be noted.
Daily Checks (outside of class time)
(Note: this information wouldn’t be given to the students because it would provide too much information about experimental design)
After the instructor notes that beetles have begun to emerge (approximately 4 weeks after oviposition), students should check their isolated beans daily and note the date of emergence and body length or mass at emergence. Typically, students measure mass at emergence because it is easier to weigh beetles than to measure body length. At the end of the experiment, students should determine emergence success.
Questions for Further Thought and Discussion
- Based on your results, have bean beetles adapted after a change in host species?
- How might the results of your experiment been different if you had used a species of phytophagous insect with greater host plant specificity? What if the insect were more of a generalist?
- Because bean beetles are an agricultural pest species, how could the results of your study be used to design an effective protocol for reducing the impact of bean beetles on stored beans?
- If phytophagous insects are able to adapt rapidly to a new host, what might this suggest about the impact of these insects in monoculture versus polyculture agricultural systems?
- Many studies have examined whether female bean beetles exhibit a preference when given a choice of several host species on which to lay their eggs. Based on the literature on host preference, how might host preference influence adaptation of bean beetles to specific host species? Contrast this with a species in which females do not exhibit a preference for host species.
References and Links
- An extensive bibliography on the ecology, evolution, and behavior of the genus Callosobruchus can be found at www.beanbeetles.org/biblio/
- Agrawal, A. A. 2000. Host-range evolution: Adaptation and trade-offs in fitness of mites on alternative hosts. Ecology 81:500-508.
- Brown, L., and J.F. Downhower.1988.Analyses in Behavioral Ecology: A Manual for Lab and Field. Sinauer Associates Publishers, Sunderland, MA.
- Carroll, S. P., H. Dingle, and S. P. Klassen. 1997. Genetic differentiation of fitness-associated traits among rapidly evolving populations of the soapberry bug. Evolution 51:1182-1188.
- Fricke, C., and G. Arnqvist.2007.Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculatus): The role of sexual selection. Evolution 61:440-454.
- Gassmann, A. J., A. Levy, T. Tran, and D. J. Futuyma. 2006. Adaptations of an insect to a novel host plant: a phylogenetic approach. Functional Ecology 20:478-485.
- Messina, F. J. 2004a. How labile are the egg-laying preferences of seed beetles? Ecological Entomology 29:318-326.
- Messina, F. J. 2004b. Predictable modification of body size and competitive ability following a host shift by a seed beetle. Evolution 58:2788-2797.
- Milanovic, D., and I. Gliksman. 2004. Selection responses and quantitative-genetic analysis of preadult performance on two host plants in the bean weevil, Acanthoscelides obtectus. Entomologia Experimentalis Et Applicata 113:125-133.
- Mitchell, R.1975. The evolution of oviposition tactics in the bean weevil, Callosobruchus maculatus F. Ecology 56:696-702.
- Mopper, S., M. Beck, D. Simberloff, and P. Stiling. 1995. Local adaptation and agents of selection in a mobile insect. Evolution 49:810-815.
- Singer, M. C., C. D. Thomas, and C. Parmesan. 1993. Rapid human-induced evolution of insect host associations. Nature 366:681-683.
- Toffolo, F. Schlyter, and S. Larsson. 2006. Host-plant use in the range expansion of the pine processionary moth, Thaumetopoea pityocampa. Ecological Entomology 31:481-490.
- Vanbergen, A. J., B. Raymond, I. S. K. Pearce, A. D. Watt, R. S. Hails, and S. E. Hartley. 2003. Host shifting by Operophtera brumata into novel environments leads to population differentiation in life-history traits. Ecological Entomology 28:604-612.
- Via, S. 1990. Ecological genetics and host adaptation in herbivorous insects - the experimental study of evolution in natural and agricultural systems. Annual Review of Entomology 35:421-446.
- Wasserman, S.S. and D.J. Futuyma.1981. Evolution of host plant utilization in laboratory populations of the southern cowpea weevil, Callosobruchus maculatus Fabrivius (Coleoptera:Bruchidae). Evolution 35:605-617.
Tools for Assessment of Student Learning Outcomes
Assessment could be carried out in a variety of ways. In the past, students have been evaluated based on a scientific paper written by each student individually. In some cases, students are evaluated on both first and second drafts of a paper, with first drafts being evaluated by a peer and by the instructor.
The scoring rubric for the papers varies with instructor. Below is an example scoring rubric used at Morehouse College for a “results summary,” which has all of the components of a scientific paper except the methods. In this evaluation rubric, “audience” concerns the choice of appropriate audience by the student. Students are expected to write their report as if it were a scientific paper. So, the appropriate audience is one of peers who have not conducted the experiment but who are scientifically literate. Reports written to the instructor or to other students in the class do not address the appropriate audience. “Format” is the overall organization of the report in sections that have parallel organization and build on each other. For example, the Discussion should evaluate the findings reported in the Results and put those results into a larger context. The Discussion also should address the hypothesis stated in the Introduction.
Results Summary Evaluation (50 points possible)
Introduction and Title Page (10 points)_____
Results (10 points)_____
Discussion and Conclusions (10 points)_____
Literature Use and Citations (10 points)_____Format, Audience (10 points) _____
Comments:
A more detailed rubric for evaluating student papers that could be adapted for this study can be found at http://tiee.ecoed.net/vol/v1/experiments/stomata/stomata_description_rubrics.html#report. In addition, a more general rubric that takes a holistic approach to evaluating student writing can be found at http://writing.umn.edu/tww/responding_grading/biologylabreport.html.
In addition to individual scientific papers, students could present the results of the experiment in the form of group scientific papers, group oral presentations, or group poster presentations. However, since all of the students are carrying out the same experiment and therefore presenting the same results, individual or group scientific papers or posters would be the most effective.
The approaches to student assessment described above are intended for use after the students complete the experiment. Students could be assessed earlier in the exercise. For example, the proposed experimental designs that students bring to class could be collected and evaluated. In addition, after the class has discussed experimental approaches, students could be asked to write a minute paper explaining how reciprocal transplant experiments can be used to test for adaptation. Both of these approaches could be used for formative evaluation as well.