Detailed Description of the Experiment
Introduction (written for students)
Diversity and the measurement of diversity are central to many issues in ecological research as well as for applying ecology to real world problems. Every textbook in ecology devotes considerable description and explanation of species diversity, species richness, and species evenness. Community ecologists use measures of diversity to study and explain ecological patterns in many different types of communities.
In terrestrial ecosystems, litter decomposition has important effects on processes such as nutrient cycling and community structure. Decomposition is affected by the type and quality of litter, climate, the edaphic conditions (including soil temperature, hydration, and chemistry), and the community of decomposer organisms (Swift et al. 1979).
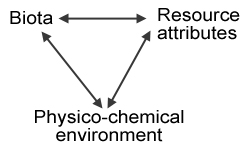
Interactions among factors that control litter decomposition (from Swift et al. 1979).
This model shows the relationships among the three factors that govern litter decomposition rates: the Biota (structure and activity of the biotic soil food webs, i.e., microbes, invertebrates, vertebrates), the Physico-chemical environment (climate, habitat, edaphic factors, i.e., contributions from the non-living environment); and Resource Attributes (primarily plant species diversity and tissue chemistry, i.e., contributions from the living environment). Many studies have shown how both the living and the non-living environments affect soil community structure and diversity (Swift et al. 1979, Elliott et al. 1980, Ingham et al. 1982, Freckman & Virginia 1997). For example, decomposition of plant litter that is high in lignin and/or low in nutrients and is therefore difficult to decompose (resource quality) leads to dominance by fungal-feeding groups in the soil food web (namely, some taxa of nematodes, mites and Collembola), whereas easily broken-down litter is decomposed primarily by bacteria, which is reflected higher up the food chain (Coleman & Crossley 1996). And soil community diversity is at least partially determined by plant community diversity (Siemann et al. 1998). So in this case, the living environment is determining the soil community. On the other hand, recent work suggests that composition and biodiversity of soil organisms itself may have a greater effect on decomposition than has been previously recognized (González and Seastedt 2001, Wardle and Lavelle 1997, Wardle et al., 2003), especially in tropical ecosystems. So in this case, the soil biota is the driving force of the Physico-chemical environment and therefore the Resource Attributes in the Swift et al. (1979) model above. On yet a third hand, the soil Biota can directly affect the Resource Attributes. De Deyn et al. (2003) showed that soil fauna enhanced succession and diversity in a grassland community.
Soil invertebrates play important roles in soil communities. Some directly consume detritus, others consume detritivores, whereas others are higher-level carnivores that can indirectly control decomposition by their effects on lower levels of the food web. The classic study of detrital food webs was conducted by Gist and Crossley (1975), showing which invertebrate groups are detritivores and which are carnivorous. This reference is somewhat hard to find; Smith and Smith (2001, p. 496) has a good description of the major findings of that study.
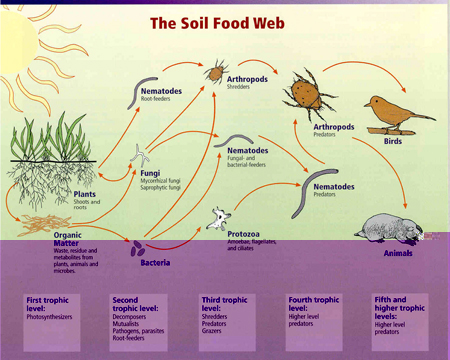
Soil invertebrates are clearly affecting litter decomposition rates, soil aeration, nutrient mineralization, primary production, and other ecosystem services related to soil ecosystem function and agroecological conservation (e.g., Six et al. 2002). With interest in global climate change has come the realization that soil biota may strongly affect soil CO2 sequestration and release, which is a critical variable in climate change models. Agroscientists and restoration ecologists have found that soil biota play critical roles in toxic chemical and metal mobility and remediation; they directly affect disturbed ecosystem recovery/ ecological restorations that occur after fire, UV-B exposure, post-urbanization, and herbicide-stressed soils (e.g., Lal 2002). Bioprospectors carry out the search for novel antibiotics and other drugs among the billions of soil microorganisms. Soil invertebrates are also recognized for their role in mediating or determining belowground interactions among plants. Because they are often prey for vertebrates such as birds and mammals, they have vital roles in the food chains that include those animals.
full size image
Relationships between soil food web, plants, organic matter, and birds and mammals. Image courtesy of USDA Natural Resources Conservation Service, http://soils.usda.gov/sqi/concepts/soil_biology/biology.html
Most students of ecology rarely have an opportunity to manipulate data sets that are self-generated and then derive diversity indices and/or graphical representations of diversity. In this class you have that opportunity, and the Soil Invertebrates Diversity Laboratory is designed to enhance your skills at calculating and interpreting diversity indices.
In order to understand gain a good background on how species diversity is determined, you should review the relevant chapters in your textbook. You can also find quite a few informative web sites. Some of the best I have found are http://www.denniskalma.com/biodiversitymeasurement.html, http://www.tnstate.edu/ganter/B412%20Ch%2015&16%20CommMetric.html, and http://www.mdsg.umd.edu/Education/biofilm/diverse.htm. The National Biological Information Infrastructure web site on this topic (http://www.nbii.gov/issues/biodiversity/) also gives some good background. This site at the University of Reading in the UK (http://www.rdg.ac.uk/ssc/software/diversity/diversity.html) even has an Excel add-in for calculating diversity indices. For additional sites, do a Google search on the words “measuring (or measure),” “species” and “diversity.”
______________________________________________________________
Materials and Methods (written for faculty)
Study Site(s):
The class will be measuring soil invertebrate density at a nearby site containing several different forest types, as well as nonforested areas. Our class has made use of the nearby St. Anne’s Convent in Melbourne, KY, near the Ohio River. We have several forest types that we can sample, including a Shumard oak (Quercus schumardii)—tulip-poplar (also called tulip tree and yellow-poplar; Liriodendron tulipifera) forest; a more mesic American beech (Fagus grandifolia) forest; a riparian forest growing along the banks of a stream; an eastern hemlock (Tsuga canadensis) forest; and several meadows.
Overview of Data Collection and Analysis Methods:
Week 1: Beforehand, the class is given some readings on factors thought to drive soil invertebrate diversity, using some of the references described in the Introduction. Either before driving to the field site, or while driving out, the class can discuss what they have read and think about what kind of questions they might test. Depending on the level of your students, you should use either the “guided inquiry” or “bounded inquiry” method for asking questions, stimulating discussion and getting students seriously started on thoughtful experiments they can answer meaningful questions. See the Notes to Faculty section for some advice in this area.
The class travels to the field site. After looking at each of the sites, student groups (2-4 students) decide what question they will ask. They will then collect samples of soil and/or litter, using bulb planters/shovels/trowels and plastic zip-lock bags. Note that the soil and litter will have very different communities, so if these are separate horizons in your soils, make sure that you separate them if both are collected.
The samples are then brought back to the lab and placed in Berlese-Tullgren funnels (see Brower et al. 1998 or http://www.albany.edu/natweb/berlese.html for good explanations). The light source is allowed to drive invertebrates into the bottom of the funnel, where they fall into alcohol or other preservative. Light bulbs of various intensities could be used to see how this affects diversity measurements. Similar volumes of soils and/or litter should be used in each funnel. With Carolina Supply funnels, I have used 100 g samples; the soils I have worked with have similar bulk densities, so this translates to a similar volume. If you are working with samples of vastly different bulk densities, such as soil from a dry site vs. a moist site, I suggest using a bulb planter to help you get similar volumes. You may need to use a larger amount than this to get good results if your samples have low invertebrate densities. With Carolina Supply funnels, I have used 60 W bulbs suspended just above the sample. With homemade funnels, you may need to use different sizes of samples. Sample collections have run for a week, as this is the interval between class meetings, but my best estimate is that invertebrates will no longer be collected after 3-5 days. I have collected the invertebrates in 70% ethanol, although apparently others have had good luck with 50-95% alcohol. I strongly suggest a “dry” run before you let the students do this.
Week 2: Students identify the invertebrates in samples, using dissecting microscopes. Invertebrates can be preserved on glass slides with drops of clear nail polish. Identification down to species is quite difficult, but identification to higher taxa and then to ecological morphotype is relatively straightforward. We identify down to class or order (or occasionally phylum) for non-insects. For insects, we identify down to order. This is done with a dichotomous key and figure (Soil Invertebrate Key; other resources include http://www.cals.ncsu.edu/course/ent591k/kwikey1.html, Edgar 1992, and Dunn and Dunn 1998). Record abundances in each sample by counting total number of each taxon or morphotype. Data are entered into a spreadsheet that can be set up to automatically calculate the Shannon index (Data Tallysheet).
Soil invertebrate biodiversity and evenness calculated using the Shannon index (H´), one of the most popular (other popular indices are described in Chapter 5b of Brower et al 1998). Shannon’s index measures both richness (the number of species) and evenness, or how evenly individuals are distributed among species. High values of H´ denote high biodiversity. Shannon’s index is advantageous over simply counting the total number of different species, because the latter is greatly affected by sampling effort (plot size and total number of individuals sampled). The greater the sample, the more rare species you find. H´ is superior because it is calculated from proportions, as you will see, and rare species contribute very little. Therefore, this index is relatively insensitive to the random inclusion or omission of rare species that happens with any sampling effort. The equation for Shannon’s index is:
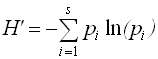
or
![]()
where the pi’s are the proportion of all observations in the ith species category, and S is the total number of species.
Shannon’s index is unitless and has no true biological meaning. However, if we take the exponent of this index, or eH´, we have an “equivalent number of equally common species.” In other words, eH´ is a type of weighted number of species present in your sample for which very common species contribute much more than do rare species to the numerical “diversity” estimate.
Consider Example 1 below. Note that although there are a total of 5 species in the sample, species A accounts for 70% of the observations. In fact eH´ for this 5 species sample is only about 2.74 “equivalently common species.” So the diversity of this 5 species community is the same as that of a community with (not quite) 3 species with all the same abundance. This community has a fairly low diversity because it is dominated by species A.
In contrast in Example 2 below, note that all 5 species are equally abundant; consequently eH´ equals 5 species. Thus, this is a high diversity community – as high as it can get for a 5 species community. In fact, the maximum value of eH´ will always be S, the number of species, when all species are equally abundant, and the actual value of eH´ should always be compared against S.
Example 1
| Species | A | B | C | D | E | Total |
|---|---|---|---|---|---|---|
| # obs. | 70 | 10 | 10 | 5 | 5 | 100 |
| pi | 0.7 | 0.1 | 0.1 | 0.05 | 0.05 | |
| H’ = -[(0.7) × ln(0.7) + 2 × (0.1) × ln(0.01) + 2 × (0.05) × ln(0.05)] | ||||||
| H' = 1.01 and eH´ = 2.74 equivalently common species | ||||||
Example 2
| Species | A | B | C | D | E | Total |
|---|---|---|---|---|---|---|
| # obs. | 20 | 20 | 20 | 20 | 20 | 100 |
| pi | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | |
| H’ = -[5 × (0.2) × ln(0.2)] | ||||||
| H' = 1.61 and eH´ = 5.00 equivalently common species | ||||||
It is also desirable to separately measure richness and evenness. A simple way to calculate richness would be the number of species. We can calculate evenness (J´) from H´. To do this, we first have to calculate the maximum possible diversity (H´max), given the number of species S. That would occur when individuals are distributed evenly among species, as in Example 2. The formula for H´max is:
H´max = ln(S)
and the formula for evenness is:
J' = H'/H´max
Thus, in Example 1, H´max = ln(5) = 1.61, and J´ = 1.01/1.61 = 0.627. In Example 2, H´max is also 1.61, but J´ = 1! That is because H´ in Example 2 is the maximum possible diversity, as the individuals are distributed evenly among species. Note that since we will usually miss rare species in a sample from a community, H´max is usually an underestimate and so J´ is an overestimate of evenness.
It is possible to carry out a t-test to compare the Shannon indices from two different communities provided that you have numerous replicates (and although it’s hard to say how many is a minimum number, a dozen samples per site would be a good target) (see p. 185 in Brower et al. 1998 or pp. 156-158 in Zar 1999). Since diversity indices are rarely normally distributed, a non-parametric test, such as the Mann-Whitney U test, or bootstrapping would be a safer bet. However, an easier, more straightforward, and more elegant way involves the non-parametric Kolmogorov-Smirnov test, which tests for differences in the trajectories of two bounded cumulative frequency distributions, which as it turns out perfectly characterizes the data you collected. Example 3 demonstrates this test.
Example 3
Two sites are sampled for 12 species. The following abundances are found:
| Number of Observations | ||
|---|---|---|
| Species | Site 1 | Site 2 |
| a | 2 | 14 |
| b | 4 | 2 |
| c | 6 | 0 |
| d | 1 | 0 |
| e | 8 | 0 |
| f | 0 | 0 |
| g | 9 | 12 |
| h | 3 | 7 |
| i | 4 | 6 |
| j | 6 | 1 |
| k | 3 | 1 |
| Total | 47 | 44 |
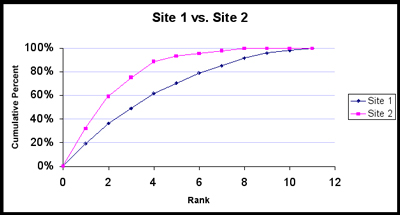
First, sort the data for each site by abundance, from highest to lowest (do this separately for each site). Note that species f is not present at either site, so it won’t be included. Calculate pi (the percentage of all observations in the ith species category) for each species, then calculate the cumulative percentages, i.e., add each pi to the sum of all preceding pi’s.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Note that we kept Species c, d and e in the computations for Site 2, because these species are present at Site 1. Now we will add a “0” to the beginning of each cumulative pi column and plot these values against each other.
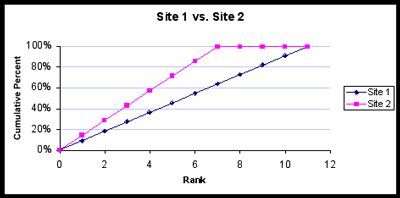
We can glean a lot of information from this plot. Site 1 is richer; notice that its curve does not reach 100% until rank 11, whereas Site 2 reaches 100% at rank 8. Also, the sharper the “knee” of the line for a community, the lower the sample diversity will be. Do you see why? This is because, if the first few ranked species account for most of the observations, the curve will shoot upward toward y = 100%, and then it will turn sharply and cruise to the right as the rare species add their puny percentages. In contrast, if the Site 1 community were perfectly diverse (all species equally represented), then the trajectory would be a straight line from (0, 0) upward and to the right until it intersects (11, 100%), as shown below. Note that the perfectly diverse Site 2 community is also a straight line until it intersects (7, 100%), as it only has 7 species.
What would happen if a site had the minimum diversity possible? This would be true if one species accounted for 100% of the individuals at a site. This is what Site 1 would look like; Site 2 would look exactly the same, since 100% of the individuals there would also be in the 1st species.
The Kolmogorov-Smirnov test is performed by taking the absolute differences of the cumulative pi’s, then taking the maximum of these differences (Dmax). For this reason, when using the Kolmogorov-Smirnov test, it is best to pool replicate samples, since your estimates of D are likely to be more precise as you collect more samples. Dmax is then compared against critical values of D (calculated by K and based on the sample sizes of the two data sets). Since the two sites will have different numbers of individuals, you will need to calculate the critical values, rather than looking them up in a table. First, calculate
![]() ;
;
for α=0.05 and 0.01. Kα(0.05)=1.3581 and Kα(0.01)=0.16276. For any α,
![]() .
.
For this example, Dcrit(0.05)=1.3581√[(44+47)/(44×47)]=0.2849 and Dcrit(0.01)=1.6276√[(44+47)/(44×47)]=0.3414. Since Dmax=0.2693, the diversities for each site do not differ significantly at P=0.05.
Calculate values of H´ and J´ for each of your sites. Also calculate the number of equivalently common species, using eH´. Then, using the procedure in Example 3, compare your values of eH´.
______________________________________________________________
Report Format
The write-up will follow the format of a scientific paper, with a Title, Introduction, Methods & Materials, Results, Discussion, Acknowledgments, and References (or Literature Cited). If you are using Pechenik (2004) in your course, be sure to read Ch. 8 before writing your report, and also pay attention to the pertinent parts of Ch. 3-5. If you are not using Pechenik or a similar guide (or even if you are), you should look at the guidelines given in the TIEE Volume 1 Stomata experiment. The write-up should be no more than 4 double-spaced pages of text (12 point font, 1-inch margins) plus and tables.
- Title: Use the title given for this lab, or another that is short and descriptive.
- Introduction: Describe the purpose and rationale of this study. This is where you should describe your hypothesis, as well as a brief summary of the background references that led you to this hypothesis. This should take no more than 1-2 paragraphs.
- Methods: Describe (briefly) the Berlese-Tullgren funnel technique that we used, as well as how we sampled soil. You may find the chapter on soil invertebrate sampling in Brower et al. (p. 110) or one of the web sites on the funnel technique (given below) to be helpful here. This should be no more than 1-2 paragraphs.
- Results: The exact number of tables and figures may vary depending upon your hypothesis. At a minimum, however, you should be reporting H´, J´ and number of equivalently common species for each site that you have included. You should also report the results of your statistical analysis; a plot of cumulative pi vs. rank should also be included. Be sure that the tables and figures follow basic formal requirements (e.g., caption, columns appropriately labeled, etc.; see Journal of Ecology or Ecology for examples), that they are as easy to read as possible, that they are in the same format, and that they are referred to in the text by number. State in the text whether any of the indices differ from each other statistically (Chapter 3 in Pechenik (2004) or the TIEE Volume 1 Stomata experiment shows how to state this in your report). Also give a brief (no more than one double-spaced page) summary of the data, which will facilitate the reader’s understanding, not just repeat what is in the tables. Point out the largest similarities and differences between the soil communities you examined.
- Discussion: In the discussion section of your lab report, address the differences (if any) that you see for invertebrates in the communities you examined. Do your results support your hypothesis(es)? Revisit the references and show how your data might or might not support what they say. Would you need further data to do this?
- Acknowledgments: Be sure to acknowledge the help of anyone who helped you. This could include your lab partner, other members of the class, the TA and/or your instructor.
- References (or Literature Cited): Be sure to use the format of a journal like Ecology for this section. You may reference this handout, the text, and any other literature, and you may also reference personal communications from the instructor or others (see Pechenik (2004) or the TIEE Volume 1 Stomata experiment for proper format). List a reference only if it is cited in the text; make sure all citations have a matching entry in the References.
______________________________________________________________
Questions for Further Thought and Discussion
- According to the indices, which community has greatest taxa diversity? Do the measures of S, H´ and J´ give different perspectives on total diversity? Why?
- Do your results suggest that litter quality drives the soil invertebrate community? Or rather, is it edaphic conditions, such as soil moisture, chemistry, etc.? Why or why not? Can litter quality and soil conditions be separated from one another in any meaningful way to answer this question? Could something else be going on? How could you design an experiment to test your hypotheses?
- Where in the soil food web do most of your invertebrates fall (what is their trophic level)? What could this be telling you about the quality of litter, richness of the site, etc.?
- There are a lot of ways in which your experimental setup could have affected your results. Remember, most of these invertebrates are small with limited mobility. What do you think you might have found if you had used a smaller amount of soil? A light bulb of a different wattage? How could you redesign your apparatus to collect a less biased sample?
- As shown in the Swift et al. (1979) model, soil communities can be affected by both the Physico-chemical environment and the Resource Attributes, where the 1st factor refers to the non-living environment and the 2nd factor refers to contributions from the living environment, namely plant tissue. There’s another way of looking at this: a site could be physically disturbed, such as by fire, trampling by large herbivores, etc., which will change the Physico-chemical environment and will certainly change the soil invertebrate community. On the other hand, the plant community will undergo succession, and this will change the resource attributes and will presumably change the soil invertebrate community. Now, both disturbance (allogenic change) and plant community change over time (autogenic change) are considered part of the process of “succession.” Can you think of a way that you could separate the two parts of succession, using soil invertebrate communities? How could you design an experiment to do this? Koehler (1998) might give you some ideas in this area.
- The composition and biodiversity of soil organisms may have a greater affect on litter decomposition than has been previously recognized. In what ways can changes in the soil invertebrate community affect litter decomposition? Both Behan-Pelletier and Newton (1999) and Hooper et al. (2000) are accessible yet well-referenced articles that can be used as a springboard for discussing this question.
- What direct effects are your soil invertebrates likely to have on large animal (vertebrates) food chains? Could the different communities you have found explain some of the differences?
______________________________________________________________
References and Links
References
- Behan-Pelletier, V., and G. Newton. 1999. Linking soil biodiversity and ecosystem function: the taxonomic dilemma. Bioscience 49:149-152.
- Brower, J. E., J. H. Zar, and C. N. von Ende. 1998. Field and laboratory methods for general ecology. 4th edition. WCB/McGraw-Hill, Boston.
- Coleman, D. C., and D. A. Crossley, Jr. 1996. Fundamentals of soil ecology. Academic Press, San Diego.
- De Deyn, G. B., C. E. Raaijmakers, H. R. Zoomer, M. P. Berg, P. C. de Ruiter, H. A. Verhoef, T. M. Bezemer, and W. H. van der Putten. 2003. Soil invertebrate fauna enhances grassland succession and diversity. Nature 422:711-713.
- Dunn, G. A., and D. K. Dunn. 1998. The insect identification guide. 4th ed. Special Publication No. 6 of the Young Entomologists’ Society, Inc. Lansing, MI. (May be ordered online at http://members.aol.com/YESbugs/pubmenu.html#Special).
- Edgar, A. L. 1992. A quantitative study of litter and soil invertebrates utilizing the Berlese funnel. In Tested studies for laboratory teaching, Volume 6. Pages 73-89 in C. A. Goldman, S. E. Andrews, P. L. Hauta, and R. Ketchum (eds). Proceedings of the 6th Workshop/Conference of the Association for Biology Laboratory Education (ABLE), 161 pages. http://www.zoo.utoronto.ca/able/volumes/vol-6/4-edgar.pdf
- Freckman, D., and R. A. Virginia. 1997. Low diversity Antarctic soil nematode communities: Distribution and response to disturbance. Ecology 78:363-369.
- Gist, C. S., and D. A. Crossely, Jr. 1975. A model of mineral-element cycling for an invertebrate food web in a southeastern hardwood forest litter community. In Mineral cycling in Southeast ecosystems, pp. 84-106. J. B. Gentry and M. H. Smith (eds.). National Technical Information Service, U.S. Dept. Commerce, Washington, DC.
- González, G., and T. R. Seastedt. 2001. Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82:955-964.
- Hooper, D. U., D. E. Bignell, V. K. Brown, L. Brussaard, J. M. Dangerfield, D. H. Wall, D. A. Wardle, D. C. Coleman, K. E. Giller, P. Lavelle, W. H. van der Putten, P. C. de Ruiter, J. Rusek, W. Silver, J. M. Tiedje, and V. Wolters. 2000. Interactions between above- and belowground biodiversity in terrestrial ecosystems: Patterns, mechanisms, and feedbacks. BioScience 50:1049-1061.
- Ingham, R. E., J. A. Trofymow, R. V. Anderson, and D. C. Coleman. 1982. Relationships between soil type and soil nematodes in a shortgrass prairie. Pedobiologica 24:139-144.
- Kalisz, P. J., and J. E. Powell. 2000. Effects of prescribed fire on soil invertebrates in upland forests on the Cumberland Plateau of Kentucky USA. Natural Areas Journal 20:336-341.
- Koehler, H. 1998. Secondary succession of soil mesofauna: A thirteen year study. Applied Soil Ecology 9:81-86.
- Lal, R. 2002. Soil carbon sequestration in China through agricultural intensification, and restoration of degraded and desertified ecosystems. Land Degradation and Development 13:469-478. http://www.environmental-expert.com/magazine/wiley/1085-3278/pdf3.pdf
- Pechenik, J. A. 2004. A short guide to writing about biology. 5th ed. Pearson Longman, New York.
- Siemann, E., D. Tilman, J. Haarstad, and M. Ritchie. 1998. Experimental tests of the dependence of arthropod diversity on plant diversity. American Naturalist 152:738-750.
- Six, J., C. Feller, K. Denef, S. M. Ogle, J. C. de Moraes Sa, and A. Albrecht. 2002. Soil organic matter, biota and aggregation in temperate and tropical soils - Effects of no-tillage. Agronomie 22:755-775. http://www.casmgs.colostate.edu/pubs/files/283_file.pdf
- Smith, R. L., and T. M. Smith. 2001. Ecology and field biology. 6th ed. Benjamin Cummings, San Francisco.
- Stamm, A., State University of New York-Oswego. 1999. Invertebrates under the snow. http://www.oswego.edu/wscp/is.htm (accessed May 11, 2004).
- Swift, M. J., O. W. Heal, and J. M Anderson. 1979. Decomposition in terrestrial ecosystems. Blackwell Scientific Publications, London.
- Wardle, D. A., and P. Lavelle. 1997. Linkages between soil biota, plant litter quality and decomposition. Pp. 107-124 in G. Cadisch and K. E. Giller, eds. Driven by nature: plant litter quality and decomposition. CAB International, London.
- Wardle, D. A., G. W. Yeates, G. M. Barker, P. J. Bellingham, K. I. Bonner, and W. M. Williamson. 2003. Island biology and ecosystem functioning in epiphytic soil communities. Science 301: 1717-1720.
- Wolters, V., W. L. Silver, D. E. Bignell, D. C. Coleman, P. Lavelle, W. H. van der Putten, P. de Ruiter, J. Rusek, D. H. Wall, D. A. Wardle, L. Brussaard, J. M. Dangerfield, V. K. Brown, K. Giller, D. U. Hooper, O. Sala, J. Tiedje, and J. A. van Veen. 2000. Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: implications for ecosystem functioning. BioScience 50:1089-1098.
- Zar, J. H. 1999. Biostatistical analysis. 4th edition. Prentice-Hall, Inc., Upper Saddle River, NJ. 931 pp.
Useful WWW Sites
- Soil preparation and measurements
http://www.dlwc.nsw.gov.au/care/soil/soil_pubs/soil_tests/soil_test_methods.html
http://www.dlwc.nsw.gov.au/care/soil/soil_pubs/soil_tests/soil_test_methods.html#table01 - US Dept of Agriculture Lab Methods Manual
http://soils.usda.gov/technical/lmm/ - Agriculture Soil Testing Lab
http://soiltest.coafes.umn.edu/methods.htm#TEXTUREANDORGANICMATTER - Recommended soil testing procedures for the Northeast US
http://ag.udel.edu/extension/Information/Soil_Testing/title-95.htm - UCSC Physiological Ecology Lab Microclimate measurements
http://www.ic.ucsc.edu/~mloik/envs162/Microclimate.htm - Soil Ecology lab (including litter, humus depth, moisture, organic matter…)
http://faculty.washington.edu/wgold/bes316%20soil%20collection%202004.doc - K-12 lab includes simple microclimate measurements
http://cires.colorado.edu/education/k12/earthworks/teachers/stevens.html - Microclimate changes in a restoration ponderosa pine site; examples of typical field measurements
http://www.envsci.nau.edu/sisklab/recent_publications/meyer_etal_2001.pdf - Onset HOBO dataloggers (temperature, light, humidity, etc.; some relatively inexpensive)
http://www.onsetcomp.com
Constructing Inexpensive Berlese-Tullgren Funnels
This is a (by no means comprehensive) list of web sites showing how you can make your own Berlese-Tullgren funnels. Many materials can be used for the funnels, including soda/milk bottle tops, inexpensive funnels, and even heavy paper. For collection, I use 70% ethanol, although I've seen others use everything from 50-95% ethanol.
- A Community Underfoot: Density and Diversity of Invertebrates in Soil or Ground Cover
This is an excellent site for constructing your own funnels
http://www.nabt.org/sub/pdf/Density1.pdf - Biodiversity Counts
http://www.amnh.org/learn/biodiversity_counts/read_select/ht/berlesefunnel.htm - Berlese Funnel Technique
http://www.albany.edu/natweb/berlese.html - Insect Collection
http://insects.tamu.edu/links/collect1.html - Detrital Food Web in Soil—shows how invertebrates interact with other soil organisms
http://www.fs.fed.us/psw/publications/documents/gtr-178/gtr-178-preface.pdf - The Soil Food Web—a nice diagram
http://www.fao.org/ag/AGL/agll/soilbiod/images/web.jpg - FAO/AGL Soil Biodiversity Portal—good reference for soil biota functions
http://www.fao.org/ag/AGL/agll/soilbiod/soilbtxt.stm - University of San Diego Excel Tutorial—Basic information, including how to use functions, filling down, formatting, inserting, graphing
http://www.usd.edu/trio/tut/excel/ - Florida Gulf Coast University Excel Tutorial—Basic information; included visuals
http://www.fgcu.edu/support/office2000/excel/ - BayCon Group Excel Tutorial—Introductory information from a commercial site
http://www.baycongroup.com/el0.htm
______________________________________________________________
Tools for Assessment of Student Learning Outcomes
There are numerous ways to assess student learning. The main tool I have used is the research report. Generally, by this point in the term, students have already had experience writing reports of this nature. I usually begin by handing out a report from a previous year, with comments, to give them an idea of what is required. I also have them read Chapter 8 in Pechenik (2003) before writing. I’ve also found it useful for student to hand in a draft that I comment upon before handing in a final report, especially if this lab is done early in the term. In order to guide both instructors and students in what constitutes a good paper, I’ve included the following rubric:
I have also used a rubric that I post for students to use. In this rubric, I have assigned so many points for each section. They can lose a certain amount of the points if they don’t include a critical component. For example, in the introduction, if they fail to propose a hypothesis, they could lose 5 out of 10 points.
In addition, students could give oral or poster presentations, with their grade based on that work.
______________________________________________________________
Tools for Formative Evaluation of this Experiment
My formative evaluation has been conducted in several ways. First, I always ask students to describe, in the Discussion of their lab report, how the lab could be improved. Unlike the case at many other schools, Ecology Laboratory at Northern Kentucky University is a separate course from the lecture, so student may or may not be taking it at the same time. Thus, at the end of the course, when students fill out a course evaluation, I specifically ask them to comment on which labs they learned the most from and which the least, and why. Finally, I collect a lot of informal feedback while we are doing the lab. For example, the rubric above was generated by student request while a lab was being carried out.
NOTE: An extensive discussion on Evaluation appears in the Teaching section of this site.