Challenges to Anticipate and Solve:
- Distinguishing between species: At times in the mixed cultures, students have difficulty distinguishing between Melittobia and
unusually small Nasonia. Preparing labeled samples of each species will help the students be able to distinguish between the
species. Oyster-eyed mutants (Carolina Biological RG 17-3425, $10.20) of Nasonia also can be used in place of the wild type
to help students distinguish between the two wasp species.
- Quantitative literacy: We have found that students have difficulty determining the values of the parameters of Lotka-Volterra
competition model. After allowing the students to discuss it in groups, the instructor may want to review the proposed calculations.
We have this discussion after the data are collected during the second lab period. However, it could take place after the consensus
experimental design is determined during the first lab period. See “Quantifying the Lotka-Volterra competition model” below for
detailed description of the calculations.
- Statistical comparisons: Students also have difficulty determining the appropriate statistical comparisons and then interpreting
the results. After allowing the students to discuss the comparisons in groups, the instructor may want to review the possible
comparisons and their interpretation. We have this discussion after the data are collected during the second lab period. However,
it could take place after the consensus experimental design is determined during the first lab period. Note: pairwise comparisons
should be made on offspring per foundress. Therefore, in treatments with two females of the same species, average number of
offspring should be divided by two prior to analysis. See “Statistical analysis of competition” below for a detailed description of the
comparisons that can be made and their interpretation.
- Culture problems: Laboratory conditions, especially during winter heating season, can be excessively dry and this may cause
cultures to desiccate resulting in low levels of emergence or high rates of culture failure, which can be frustrating to students. It is best
if cultures can be maintained in an incubator (about 260 40-60%RH). Under poor culture conditions, as high as 50% of cultures may fail.
The highest failure rate is with cultures with single foundresses. As a result, we recommend establishing a minimum of 20 replicates.
Those cultures that do not produce any offspring should be removed from analyses.
______________________________________________________________
Comments On the Lab Description:
The “Detailed Description of the Experiment” above is intended as a student handout. Although all of the students ultimately will
perform the same experiment, the student handout is designed to lead students through the process of experimental design and
analysis of data. As a result, we have intentionally left out details on the exact experimental design and analysis of data. Those
details are presented here for instructors. They could be inserted into a student handout; however, in our experience, giving students
the details up front leads students to think that there is only one correct approach to the question. In addition, we use this laboratory
exercise after we have discussed competition, including the Lotka-Volterra competition model, in lecture. As a result, we do not
present a detailed discussion of the model and all possible outcomes in the student handout. The details that are presented are
intended as a reminder for students of what was covered in lecture already. However, if the exercise is used independent of a lecture
course or before discussion of competition in lecture, a more detailed discussion of the Lotka-Volterra competition model may need
to be included in the student handout.
Introducing the Lab to Your Students:
Because this is a guided inquiry, after each student group has developed their list of possible interaction experiments, the instructor’s role
should be to moderate the sharing session during which each group will present their ideas for experiments. Make suggestions or ask
leading questions as dictated by the class dynamics to lead the class to develop a set of logical investigations. Attempt to involve members
of every group in the discussion and avoid letting one student or group dominate.
On the board or overhead projector, set up a table with four columns. In the first, help students think through the important experimental
questions. In column two, develop a running list of various possible treatments that address the experimental questions in the first
column. Help students see that this should be various combinations of the two wasps. In column three, for each possible experimental condition list
specific predictions of anticipated outcomes proposed by your students. Accept all predictions students make about the outcomes at this
time, but allow student generated discussion concerning them. In column four, elicit their prediction for how the relative numbers of the
offspring of each species will change compared to when each is alone on a host, assuming that competition is present.
Set up a second table to develop lists of variables to be kept constant or controlled in each experiment. Encourage student brainstorming
on this topic until it seems that all relevant matters have been addressed.
Have students copy these two tables and submit them as part of their laboratory report at the close of the investigation.
Samples of Student Thinking About the Experimental Set-Up
|
| Nature of the question |
Treatments - # of parasitoid(s) on a host |
Specific predictions |
| Types of interactions |
Effect on offspring number |
| What is the reproductive potential for a female Melittobia without competition? |
One Melittobia |
“This will be the highest number because these wasps are smallest so more of them fit.” |
N/A |
| What is the reproductive potential for a female Nasonia without competition? |
One Nasonia |
“There will be fewer of these because they are larger, but more of them than when they have to
share a host with another wasp.” |
N/A |
| Is the outcome of the interspecific interatction competition, neutral, commensalism, or mutualism? |
One of each species |
“I think they’ll share the host, one taking the head and the other the tail end.” |
“There will be slightly more Melittobia than Nasonia, but the total will not be greater than either
species alone since the host is a finite amount of food.” |
| Is the outcome intraspecific interaction in Melittobia competition, cooperation, or neutral sharing
of the resource? |
Two Melittobia |
“There will be fewer offspring per female because they will be crowded together.” |
“The total number of offspring will be the same as with one female by herself since the host is
a finite amount of food.” |
| Is the outcome intraspecific interaction in Nasonia competition, cooperation, or neutral sharing
of the resource? |
Two Nasonia |
“They’ll fight each other and end up with only one alive to lay eggs.” |
“The total number of offspring will be the same as with one female by herself since the host is a
finite amount of food.” |
| Which is more important, intraspecific or interspecific competition? |
Two of each species |
“Because Nasonia are larger they should be better interspecific competitors. But Melittobia
produce more offspring, so intraspecific competition will be more important.” |
“The total number of offspring of each species will be the same as with just one of each species,
if interspecific competition is most important.” |
Ultimately guide students to appreciate that the most complete way to investigate and understand the possible interactions between
two wasp species competing for a single host resource would include the following four treatments.
1. A single female alone on a host (Treatment 1 - one for each species)
2. Two females of the same species on a host (Treatment 2 - one for each species)
3. A female of each species together on a host (Treatment 1+1)
4. Two Melittobia and two Nasonia females together on a host (Treatment 2+2)
Treatment 1 will show the reproductive potential for each female in the absence of competition between foundresses. Treatment 2 will show
if two females sharing a single host (intraspecific competition) produce more or fewer offspring as compared to when they have sole
possession of a host (treatment 1). Treatment 1+1 will reveal whether one species is able to outcompete the other for a single limiting
resource (interspecific competition) or whether some form of sharing occurs. Treatment 2+2 will demonstrate the interaction between
interspecific and intraspecific competition. For example, a comparison of Treatment 2 (intraspecific competition) with Treatment 2+2
(both intraspecific and interspecific competition) will suggest the importance of interspecific competition when intraspecific competition is
present (see Statistical analysis of competition, below). This is also a good opportunity to discuss the need for developing testable
predictions. For example, although the student’s third and fifth predictions in the table above might be possible outcomes, given the
structure of this experiment, they are impossible to evaluate.
Comments On the Activities in the Lab.
Control of variables:
To control for possible host effects, there are at least two considerations that should be discussed and agreed upon prior to starting
the experiment. First, fly host weights vary rather greatly, with the larger (ca. 0.125g) being more than twice the weight of the smaller
(ca. 0.055g). Such variation can obviously affect the potential number of parasitoid progeny, with lower yields from smaller hosts
compared to larger hosts. Lead students to consider the importance of weighing the hosts and using relatively uniform host sizes for
all experiments. Alternatively, they could calculate a conversion or adjustment factor, i.e., average number of progeny per milligram of
host and adjust their data accordingly (see “Other Extensions,” below).
Note: an interesting extension would be to run one set of treatments on the largest size hosts and a parallel set on the smallest size
hosts to explore whether host weight changes the results in a consistent or predictable fashion. There is evidence that host size
influences the outcome of intraspecific competition in Melittobia (C. Randall and J. Guinan, unpublished data).
Handling techniques:
Prior to having the students set up their individual or group experiment, demonstrate how to remove a few wasps onto a piece of white
copy paper, by gently brushing them with the side of a pipe cleaner. Demonstrate how to use an inverted shell vial to readily capture
one, which will immediately crawl up into the vial. Finally, and this is critically important, make a big deal about tightly plugging the
vials with a cotton ball once the wasps and host are inside. Loose cotton plugs will result in escaped wasps and experiment failure.
Discuss with students the matter of how to label their experimental vials, and have them write legibly.
The treatments should be stored in an upright position. An excellent way to organize and store the vials is to use the box in which they
were sent. It contains dividers that will hold the vials in an upright position. If the box is placed in a convenient drawer, students can
have easy access to check the progress of their experiment. Another option is to purchase heavyweight cardboard vial trays that will
store up to 112 cultures upright (Carolina Biological Supply, ER-71-4906, $4.50 each).
Conducting the Investigation:
Part One. Once everyone has agreed on the treatments to be used and the appropriate protocols, students can be directed to the materials
table to initiate the experiment. Because the materials are relatively inexpensive, each student can be responsible for conducting one
replicate (a total of five vials). Alternatively, replicates can be divided up so that each group is responsible for one replicate. The former is
recommended, however, as having more replicates increases the confidence in the results and also helps mitigate against the occasional
experiment failure or unforeseen disaster.
At least once a week over the next four weeks, have students briefly examine their cultures, noting any changes that are evident. This should
take only a few moments, and should not interfere with other laboratory activities you have scheduled.
Part Two. It is best not to schedule the lab for the second half of this experiment for at least 5 weeks after the students have established
their cultures. Four or five weeks after initiating the experiment, the new generation of Melittobia adults should be emerged. For cultures only
containing Nasonia foundresses, emergence will take about half as long. Several days after the adults have emerged, you should collect
the vials into a resealable plastic bag and place the bag in the freezer compartment of a refrigerator until class. This will serve to euthanize
remaining live wasps and keep all of them relatively soft and pliable so they can be counted more easily.
To test validity of their predictions, students will need to count the total number of adult wasps produced in each treatment. Consider also
having students maintain records of the sex and body size of the offspring (see “Other Extensions,” below.) Comparing the pooled class
results for each of the treatments will lead to conclusions about the nature of the interaction.
When it comes time to examine the offspring, suggest that students empty the contents of their experimental vial onto a piece of white copy
paper. They can then use a pipe cleaner to move the dead wasps into small groups for tallying totals. Caution them to exercise care during
counting. Wasps are easily lost if the student sneezes or breathes heavily on them. Also remind them that because some wasps will die
inside the host pupa skin, it will be necessary to break open the host remains and brush out any wasps remaining inside. In some cases,
students may find larvae or pupae as well as adults. It is probably best not to include them in the counts. Some of these may not be viable
and would never have emerged. In addition, sex is impossible to determine in larval and in the early pupal stage, so if your students are
keeping track of sex ratios, they would not be able to classify these offspring.
When the students’ experiments have been concluded, class results can be pooled onto a spreadsheet, with copies made available for each
student. Results from multiple class sections may also be compiled to provide larger numbers of replicates.
Quantifying the Lotka-Volterra Competition Model:
First, it is important to note that traditionally the Lotka-Volterra competition model has
been applied to systems in which the resource for which species are competing is renewable such that multiple generations can use the
resource. However, in this experiment, only a single generation of wasps can be produced because the host is not a renewable resource.
As a result, this experiment allows us to examine whether the Lotka-Volterra model can accurately predict the outcome of competition for
a non-renewable resource.
To quantify the Lotka-Volterra competition model, students must determine the carrying capacities and competition coefficients for both
species. We allow students to work in groups to determine which treatments should be used to estimate these values and then discuss
their ideas as a class. Remind the students that estimates should be based on treatment averages for the pooled data and not on just their
individual replicates. Also, if students are dividing offspring by sex, parameter estimates should be based on the total number of offspring,
because of female-biased sex ratios, especially in Melittobia.
To estimate the carrying capacities for each species, it is critical that the entire host is consumed by the parasitoid larvae. In some cases,
single foundresses may not produce sufficient offspring to consume an entire host. On their natural hosts, the first Melittobia females that
hatch are non-dispersing, which suggests that the host can be used by multiple generations (Freeman and Ittyeipe 1976, Côsoli and Vinson
2002). Therefore, carrying capacities are best estimated by the total number of offspring produced with two foundresses (i.e., 2M or 2N
treatments). It is important to emphasize that in determining the carrying capacities, we are interested in the total number of adult offspring
that can be produced on a given host and not the number of offspring per foundress.
When estimating the competition coefficients, we are interested in the effect of interspecific competition alone. As a result, the 1+1 treatment
should be used to determine the values of N1 and N2 needed to estimate the competition coefficients.
Carrying capacities also are needed to
estimate the competition coefficients. Therefore, we discuss estimating carrying capacities first. In the 2+2 treatment, both intraspecific
and interspecific competition are occurring, and intraspecific competition can be strong enough to limit the effects of interspecific competition.
Therefore, it is not appropriate to use the 2+2 treatment for estimating the competition coefficients.
Below is an example based on data from an Emory University ecology class that is present below.
KN = 31.8
KM = 132.9
aNM = (KN - NN)/ NM = (31.8 - 18.1)/6.7 = 2.04
aMN = (KM - NM)/ NN = (132.9 - 6.7)/18.1 = 6.97
In all of the trials that we have conducted, the estimates of the parameters of the Lotka-Volterra competition model suggest an unstable
coexistence between the two species. As a result, the outcome of competition will depend on the initial densities of the two species.
In interpreting the results of the Lotka-Volterra competition model, students often think that the number of foundresses of each species
represents the initial densities of the two species. It is important to emphasize that it is the larvae that are competing for the resource.
Because an unstable coexistence between the two species is typically predicted based on the data, a possible extension of the experiment
is to vary the number of foundresses of each species independently (e. g., 1M+2N, 2M+1N). However, it is important to keep in mind that an
increase in the number of foundresses may not necessarily lead to a proportional increase in the number of competing larvae, as parasitoid
are known to adjust their clutch size based on the presence of conspecific and heterospecific offspring (e. g., Werren 1984,
Mackauer et al. 1992).
Statistical analysis of competition:
The experiment is designed to permit students to examine the effect of both intraspecific and interspecific
competition on offspring production (male, female, total) using planned statistical contrasts. To understand the contrasts, we have the
students first identify what type of competition, if any, is occurring in each treatment. Then, we ask students to determine what particular
comparisons of pairs of treatments tell us about competition. Below are the treatments and comparisons and how they relate to competition.
We would not give these tables to students, but ask them to generate the tables themselves.
| Treatment: | Type of Competition: |
| 1 foundress (Trt 1) | No competition between offspring of different foundresses |
| 2 foundresses of the same species (Trt 2) | Intraspecific competition |
1 foundress of each species
(Trt 1+1) | Interspecific competition |
2 foundresses of each species
(Trt 2+2) | Intraspecific and interspecific competition |
| |
| Contrast: |
What it tells us: |
| Trt 1 vs Trt 2 |
Strength of intraspecific competition |
| Trt 1 vs Trt 1+1 |
Strength of interspecific competition |
| Trt 1 vs Trt 2+2 |
Strength of combined competition |
| Trt 2 vs Trt 1+1 |
Relative strength of intraspecific and interspecific competition |
| Trt 2 vs Trt 2+2 |
Relative strength of interspecific competition in the presence of intraspecific competition |
| Trt1+1 vs Trt 2+2 |
Relative strength of intraspecific competition in the presence of interspecific competition |
Since all of the contrasts are between two treatments, t-tests can be used for all of the analyses. The analysis can be done using data on
offspring production or offspring production per gram host mass (see “Variation in host mass” under “Other Extensions,” below). In either
case, offspring production should be expressed per foundress before analysis. In treatments with more than one foundress of a particular
species, we cannot determine which foundress produced the offspring. Therefore, we assume that offspring production was equal for each
foundress and just divide the number of offspring produced by the number of foundresses. It is also important to note that we are assuming
no (or limited) competition when there is only a single foundress. Currently, it is unknown whether competition is occurring among offspring
of a single foundress, as the number of eggs laid has not been determined. How host size and number of foundresses affects number of
eggs laid also is unknown. However, in Melittobia on their natural hosts, the first females that hatch are non-dispersing, which suggests
that the host can be used by multiple generations (Freeman and Ittyeipe 1976, Côsoli and Vinson 2002) and that host resources are not
limited for the first generation.
Sample of expected results:
Interspecific Competition. At the University of Georgia, we have run nearly 600 trials in sets of 100 replicates placing one female of each
species with a single host pupa at 26oC with the following general outcomes:
* Only Nasonia vitripennis results: 30-36%
* Only Melittobia digitata results: 22-27%
* Each produce some offspring: 24-33%
* Neither produce any offspring: 7-15%
Intraspecific Competition. The table that follows lists outcomes of research on different numbers of Melittobia and Nasonia alone on a
single host fly pupa. Although the activity, as written, does not include sex ratio data, we’ve included it here in case you wish to make
this an optional addition for more advanced classes or extra credit.
| Sample Outcomes of Studies of Competition Between N. vitripennis and M. digitata on the
Same Neobellierria Host |
| Number of Mothers | Sons |
Daughters | Total Progeny |
Sex Ratio
(% males) | Sample Size |
Source |
Nasonia vitripennis |
| 1 | 10.0 |
54.6 | 64.6 |
16 % | 10 |
B. King 2000 |
| 2 | 24.6 |
34.1 | 58.7 |
43 % | 9 |
Melittobia digitata |
| 1 | 3.1 |
93.7 | 96.8 |
3.2 % | 11 |
Silva-Torres and Matthews, 2003 |
| 2 | 4.2 |
128.4 | 132.6 |
3.2 % | 16 |
Both Nasonia vitripennis and Melittobia digitata |
| 1 N. vitripennis | 6.8 |
9.3 | 16.1 |
42.2 | 146 |
Matthews, unpublished |
| 1 M. digitata |
0.7 | 7.1 |
7.8 | 8.97 |
146 |
| 2 N. vitripennis |
16.9 | 18.6 |
35.5 | 44.4 |
44 | West, unpublished |
| 2 M. digitata |
0.02 | 1.34 |
1.36 | 1.0 |
44 |
Conclusions:
Interspecific competition. A single individual either species alone with a host produces significantly more progeny than it does
even if it wins in an interspecific competition situation. When both produce some progeny in the competition, the total production per
species decreases even further. Thus the presence of a competitor seriously impacts reproductive success (fitness). One can also
speculate about whether the relative sizes of the two competing species might be a factor in the outcome, given that Nasonia require
about twice as much host resource per offspring as do Melittobia. Other possible topics for discussion include the effect of differing
generation times between Melittobia and Nasonia, and the fact that blowflies are not the natural host of Melittobia,
but are for Nasonia. Also, females of both species feed on host fluids. Thus, the greater the number of females,
the greater an effect they have on depletion of the host resource. Finally, there is also the possibility that venom or other
chemicals injected into the hosts at the time of initial parasitoid attack alter the host’s physiology in ways that affect the outcome
of the competition. This aspect is so far totally uninvestigated, but could be a topic for speculation.
Intraspecific competition. In Melittobia digitata, the total number of progeny is higher with two foundresses, but the number of progeny
per foundress when two foundresses are placed with a host decreases (Cooperband et al. 2003, Silva-Torres and Matthews 2003).
However, one big unknown, and a serious limitation of the data, is that we are unable to know the relative contributions of the two
individual wasps in a competitive context. We assume that both are equivalent and infer that the reduced per capita output is a result
of competition. Students should be led to think critically about assumptions being made in any experiment. In addition to offspring
number, competition apparently affects offspring size and viability. Forewing length and hind tibia length of female progeny from single-female
cultures are larger than those from cultures where two or more females are together on a single host. Also, progeny from single female
experiments live significantly longer (Silva-Torres and Matthews 2003).
In Nasonia vitripennis, two females on a host produce slightly fewer total progeny and the number per female (per capita rate) is considerably
lower compared to a single female alone. Interestingly, the sex ratio also changes dramatically, with the proportion of males being much
greater when two females share hosts (Werren 1983, King 2000). However, having more than one female Melittobia in the initial set-up does
not change the sex ratio of the offspring from that found with a single female (Abe et al. 2003, Cooperband et al. 2003, Silva-Torres and
Matthews 2003). Attempting to understand such differences leads into the fascinating area of local mate competition theory and how
differences in the life histories and mating behaviors define behavioral expression in the two species.
Distinguishing between the species: Because adult Nasonia can be small, especially when there is competition for host resources,
the two species cannot always be distinguished by body size. Therefore, it is important to point out to students the characters that can
be used to distinguish between the species. The most reliable characters are head shape and body shape. Nasonia have a distinctly
round head and Melittobia a flattened and elongated head when viewed from the side (see figure below). The thorax and abdomen are about the
same thickness in Nasonia. In contrast, in Melittobia, the thorax is thinner than the abdomen when viewed from the side.
 |  |
| Nasonia vitripennis | Melittobia digitata |
| ___________________________ |
 |
| Nasonia vitripennis (left) and Melittobia digitata to show typical size differences |
| (photos © Jorge M. González) |
Distinguishing between females and males:
Melittobia males and females are easy to tell apart. Females have straight dark bodies, straight antennae, and fully-developed
wings. Males are amber colored, have branched antennae, and stunted wings (see figure below).
 |
| Sexes of Melittobia digitata, with a female at left and a male at right. |
| (photos © Robert W. Matthews) |
Nasonia sex identification is a little trickier, but students in advanced classes can learn to do it with practice.
The most reliable difference between the sexes is that males have stunted wings, while females’ wings are fully developed.
It’s important to stress to students that size is not a reliable indicator of sex, as some of them might assume otherwise. If you are planning
to have students tally male and female offspring separately, it is also helpful to prepare separate labeled vials containing a single male and
female (a few vials for Melittobia, some for Nasonia). One vial for each species can be handed out to each group. Students can
examine these specimens under a dissecting scope while you explain how to differentiate between the species and the sexes within each
species. This can be done in the first lab session, or you can wait until part two of the lab, when students will be tallying the results. In the
latter case, place the vials in the freezer until they are needed.
Maintaining parasitoid wasp cultures:
Maintaining your own stock cultures of wasps is an easy and inexpensive way of producing large quantities of wasps when you need them.
To maintain a culture, simply place 3-4 hosts in a clean, 1-dram vial, along with 5-6 mated females (almost all should be mated within 24
hours of emerging as adults), and close tightly with a cotton ball plug. The wasps will mature more quickly in an incubator set at about
25-26oC, but can be raised at room temperatures as well. Melittobia should emerge in about 18-28 days, and Nasonia
in about 14 days. The easiest way to ensure that you have enough mated females available when you need them is to stagger the setup of your
cultures. For Melittobia, begin by establishing two cultures (in case one fails for some reason) about 32 days before you’ll need them, and
establish additional cultures every 3 days or so for about 10 days. Each culture will produce at least 300 females, so you’ll have far more females
than you need, but as the cultures are so inexpensive to set up, you’ll be sure to have enough young females to use for the lab. For
Nasonia, start about 20 days in advance and establish cultures every 2-3 days for a week. Each Nasonia culture should yield about
50 wasps per host.
Other Extensions:
Although the experiment is intended for students to investigate the Lotka-Volterra competition model, the experiment can be extended or
adapted to examine other related questions.
Variation in Host Mass.
In the general protocol, students are provided with hosts that are greater than 0.1g. However, the hosts still may vary considerably in
mass. As a result, students could consider the effect of host mass. To do so, students weigh the hosts prior to the initiation of the
experiment. With data on host mass, students can examine the effect of host mass on offspring production (male, female, and total) in
each treatment by plotting offspring number versus host mass and carrying out a linear regression analysis. In addition, students can
control for host mass in their analysis of the effects of competition by dividing the number of offspring produced per female by host mass
for each replicate prior to analysis (see “Statistical Analysis of Competition,” above). The importance of host mass could be explored to
an even greater extent by using a wider range of host masses, rather than limiting hosts to those greater than 0.1g.
Effect of Competition on Offspring Quality.
In addition to affecting offspring number, competition can influence offspring quality. Students can determine offspring quality by
measuring body size in a subset of offspring from each replicate. For the species used in this study, wing length, head width, or
hind tibia length are often used as a measure of body size. These can be determined by using a dissecting scope equipped with
an ocular micrometer. Because Melittobia males have significantly larger heads than females (C. Randall and J. Guinan, unpublished data),
students should analyze the data for males and females separately or ignore the males. Students can investigate the effects of host size
and competition on offspring quality itself by using the analyses described above. In addition, students may want to determine the
relationship between offspring number and offspring quality for each treatment, by using linear regression with offspring number as the
independent variable and offspring quality as the dependent variable. If offspring number does significantly affect offspring quality, then
students could examine the effects of host size and competition on offspring quality after controlling for the effects of offspring number.
Perhaps the easiest way to do this is to save the residuals from the regression of offspring number and offspring quality and then
analyzing the residuals as described above. The residuals describe the variation in offspring quality that is not explained by variation
in offspring number.
Effect of Invasion Sequence.
In interspecific competition treatments (1+1 or 2+2), the experimental protocol calls for students to introduce foundresses of both species
into the culture at the same time. However, if the two species were to use the same host in nature (remember that they don’t), it is unlikely
that both species would find the host at the same time. As a result, students could investigate the effect of invasion sequence by
staggering when foundresses are introduced.
Effect of Female Number on Sex Ratio.
Published data for sex ratio adjustment in Nasonia and Melittobia differ strikingly. For Nasonia, presence of multiple females on a host
results in an increased proportion of males (Werren 1983, King 2000), whereas for Melittobia the sex ratio remains constant with increasing
numbers of females (Abe et al. 2003, Cooperband et al. 2003, Silva-Torres 2003). Careful counts of the sexes produced in each treatment
would allow students to confirm whether these trends hold under different treatments. It is noteworthy that Melittobia males also engage in
lethal combat, and the extent of this could be partly assessed by simply tallying the numbers of intact vs. dismembered males in treatments
where Melittobia emerged. Male fighting is postulated to be related to the apparent failure of Melittobia to conform to predictions from
local mate competition theory (Abe et al. 2003).
______________________________________________________________
Comments On Questions for Further Thought:
Although the questions for further thought are included in the student handout, most of us do not have the students answer the questions
explicitly, as the students are required to write a scientific paper based on the results of the experiment. The questions would be most
appropriate if students are not required to submit a written report. Below are comments on expected answers.
Comment on Question 1:
Based on the parameter values that you calculated for the Lotka-Volterra competition model, what is the predicted outcome of
competition between the two species? Was the predicted outcome achieved in every replicate of interspecific competition?
If not, why not?
In all trials that we have run, the predicted outcome of competition is an unstable coexistence. In some replicates of one
Nasonia and one Melittobia, one species competitively excludes the other, as predicted by the Lotka-Volterra
model. However, in many replicates, we see both species coexisting. The difference between the prediction of the model and
the actual outcome may be due to the fact that the model is most appropriate for systems in which multiple generations can
feed on the same renewable, but limited, resource, which is not possible in this system. In addition, the parameter estimates
for the Lotka-Volterra model are point estimates and do not consider the variation across replicates.
Comment on Question 2:
“Gause’s Law” states that competitors that share exactly the same resources in the same way cannot coexist. This means that the
species that most efficiently uses the contested resource will eventually eliminate the other at that location. Does Gause’s Law seem
to apply to the interaction between Nasonia and Melittobia? Why or why not?
We have asked this question in introductory biology courses. The responses of students seem to depend on whether students consider
trends in the data as a whole or whether they consider individual replicates independently. If they consider the trends in the data as a
whole, they find that Gause’s law holds in that Nasonia tends to exclude Melittobia in interspecific competition.
However, if they consider each replicate, they state that Gause’s law does not hold, because there is coexistence in some replicates.
In a very few cases, students consider coexistence as evidence that the two species are not complete competitors.
Comment on Question 3:
If these two species were to use the same host in nature, how might resource partitioning allow them to coexist?
Since the two species use the same life cycle stage of the host, they could only partition the host resource by using different parts of the
host, rather than different life cycle stages.
Comment on Question 4:
Based on the results of your experiment, why don’t the two species use the same host in nature?
The results suggest an unstable coexistence, in which one species is excluded. As a result, both species cannot coexist on the same
host in nature. Therefore, if the two species competed for the same host early in their evolutionary history, they have since diverged in
what host species they use to avoid competition (niche partitioning or ecological character displacement).
Comment on Question 5:
Given the estimated values for carrying capacities and competition coefficients, predict the outcome of competition between
Melittobia and Nasonia using the Lotka-Volterra competition model in Populus
(see References and Links).
Is the predicted outcome of competition affected by initial population sizes or population growth rates? If so, how? How is the time
to reach equilibrium affected by these values?
As stated above, the predicted outcome is unstable coexistence. In this case of the Lotka-Volterra model, the outcome of competition
is affected both by initial population sizes and by population growth rates. In general, the species with the larger initial population size
and higher population growth rate will competitively exclude the other species. In all other cases of the model, the outcome of competition
is not affected by either initial population size or population growth rates. Larger initial population sizes and higher population growth
rates will lead to a decreased time to reach equilibrium.
Comment on Question 6:
The carrying capacities and competition coefficients are just estimates. What factors might affect the carrying capacities and competition
coefficients for these two species?
Carrying capacities and competition coefficients can be affected by a variety of factors, including host quality, initial population densities of
competitors, characteristics of the founding populations of competitors, and environmental conditions such as temperature and humidity.
Comment on Question 7:
If interspecific competition occurs in these species, how might we determine what mechanism of competition (interference or exploitative)
is occurring?
In this system, it would be very difficult to prevent interference competition among larvae to examine the effects of exploitative competition
among larvae alone. Therefore, direct observations on competing larvae would be necessary to determine whether interference competition
is occurring.
However, Hawkins (2000) suggests that all competition among larvae is interference. Exploitative competition among parasitoids occurs
when one adult parasitoid attacks or kills a host before another parasitoid, thus limiting the availability of the host to the later parasitoid.
Adult female parasitoids also can engage in interference competition while searching for hosts (Hawkins 2000). By examining the effect
of invasion sequence, students could determine the effect of competition among adults.
______________________________________________________________
Comments On the Assessment of Student Learning Outcomes:
Details of the assessment methods are presented in the
"Description: Tools for Assessment of Student Learning Outcomes” section.
Assessment of student learning in this experiment has been evaluated in two different ways. At Morehouse College, students
are given a pre-test and post-test over the range of subjects taught in general ecology. At Emory University and Radford
University, students are given a pre-test and post-test specifically on interspecific competition (see below). Student
performance on the two tests is then compared.
At Morehouse College, there was a significant improvement on the post-test as compared to the pre-test. However, the degree of
improvement was not influenced by whether students were enrolled in the laboratory or not. Yet, since the assessment does not
address just competition, we cannot draw specific conclusions about this exercise.
At Emory University, in one semester, students exhibited significant increases in overall score and in scores on questions
related to the Lotka-Volterra model (Q3 and Q4) on the post-test as compared to the pre-test (paired t-test, one-tailed P < 0.5).
In another semester, there were no differences between pre-test and post-test scores. Students at Radford University exhibited a
similar pattern with significant differences between pre-test and post-test scores in one semester, but not in another.
An example student assessment instrument:
Assessment for Melittobia - Nasonia Competition Lab
- Gause’s competitive exclusion principle states that:
- intraspecific competition is always stronger than interspecific competition,
- interspecific competition is always stronger than intraspecific competition,
- two species cannot occupy the same ecological niche,
- two individuals of the same species cannot occupy the same ecological niche,
- both a. and d. are correct.
- For competition to occur, what must be true about resources in the environment?
- Two very similar species, ditzy-headed dingbats and nasty-tempered meanbats, which use the same limiting resource,
are introduced into the same area. When a dingbat has a habitat all to itself, it can produce 60 offspring a year.
When two dingbats share a habitat, they each produce an average of 30 each offspring a year. A solitary meanbat
can produce 50 offspring per annum, while two meanbats sharing a habitat produce only 25 offspring each per year.
However, when a ditzy-headed dingbat and a mean-tempered meanbat share a habitat, the dingbat produces just 25
offspring and the meanbat produces 40 offspring. Answer the following questions regarding this interaction:
Which type of competition is stronger for dingbats, intra- or interspecific competition? Explain your answer.
Which type of competition is stronger for meanbats, intra- or interspecific competition? Explain your answer.
Using dingbats as species 1 and meanbats as species 2, calculate alpha12 of the Lotka-Volterra equation.
- If the graph of the Lotka-Volterra equation for this interaction is as shown below, what will be the probable outcome of this interaction?
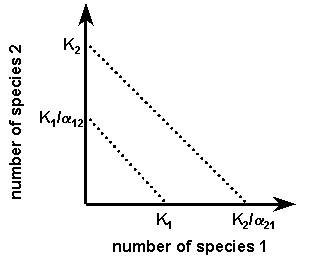
- dingbats will exclude meanbats,
- meanbats will exclude dingbats,
- either species will eventually exclude the other,
- the two species will coexist.
|
______________________________________________________________
Comments On the Evaluation of the Lab Activity:
______________________________________________________________
Comments On Translating the Activity to Other Institutional Scales:
A version of this experiment has been implemented successfully in an introductory biology course for non-majors at a large public
university by emphasizing qualitative comparisons of the effects of intraspecific and interspecific competition. The version for introductory
biology was presented as a major workshop at the annual meeting of the Association for Biology Laboratory Education (ABLE) in 2004
(www.zoo.utoronto.ca/able/conf/able2004/abstracts.htm)
and will be published in the proceedings of the conference in June 2005. This version of the exercise does not include examination of the
Lotka-Volterra competition model, but involves more qualitative analysis of the results. Prior to publication in the proceedings, the
version for introductory biology is available from the authors.






